
Dr. Chandi Charan Malakar @ NIT Manipur (Chemistry)
Welcome to the Synthesis and Catalysis Research Group

National Institute of Technology Manipur
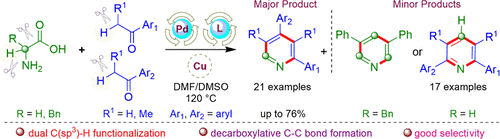
(87) R. Gujjarappa, N. Vodnala, D. Musib, and C. C. Malakar
“Organocatalytic Decarboxylation and Dual C(sp3)-H Bond Functionalization Toward Facile Access to Divergent 2,6-Diarylpyridines”
Asian J. Org. Chem. 2022, https://doi.org/10.1002/ajoc.202100627
(86) M. Singh, R. Jamra, A. K. Paul, C. C. Malakar, V. Singh
“KI-assisted Sulfur Activation/Insertion/Denitration Strategy towards Dual C−S Bond Formation for One-pot Synthesis of β-Carboline-tethered 2-
Acylbenzothiophenes”
Asian J. Org. Chem. 2022, https://doi.org/10.1002/ajoc.202100653
(85) A. Garg, K. Kant, K. K. Roy, A. Sahoo, C. C. Malakar, S. Gupta
“Docking-based evaluation against Human Tankyrase-1 and Tankyrase-2 enzyme”
Materialstoday: Proceedings. 2022, https://doi.org/10.1016/j.matpr.2022.03.095
(84) R. Reetu, A. Garg, K. K. Roy, A. Roy, S. Gupta, C. C. Malakar
“In-silico studies for targeting PPARγ for the Type II Diabetes Mellitus”
Materialstoday: Proceedings. 2022, https://doi.org/10.1016/j.matpr.2022.01.299
(83) R. Reetu, R. Gujjarappa, C. C. Malakar
“Recent Advances in Synthesis and Medicinal Evaluation of 1,2-Benzothiazine Analogues”
Asian J. Org. Chem. 2022, https://doi.org/10.1002/ajoc.202200163
(82) A. K. Kabi, M. Pal, R. Gujjarappa, C. C. Malakar, Mithun Roy
“Overview of Hydroxychloroquine and Remdesivir on severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)”
J. Heterocycl. Chem. 2022, https://onlinelibrary.wiley.com/doi/full/10.1002/jhet.4541
(81) E. P. Devi, D. Kaldhi, R. Sengupta, A. Roy, A. Sahoo, I. Saha, C. C. Malakar
“Catalytic Iodine and Morpholine as Reagent Combination for Hydration of Alkynes via Markovnikov Addition”
Asian J. Chem. 2022, DOI: 10.14233/ajchem.2022.23751
(80) L. S. Singh, A. K. Kabi, R. Sengupta, A. Roy, A. Sahoo, I. Saha, C. C. Malakar
“C-H Functionalization and C-N Bond Formation Approaches under Catalytic Conditions for the Synthesis of α-Ketoamides and 2,4-Disubstituted-1,3,5-triazines”
Asian J. Chem. 2022, DOI: 10.14233/ajchem.2022.23769
(79) A. K. Kabi, S. Sravani, R. Gujjarappa, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, R. Velayutham, S. Gupta,
C. C. Malakar
“An Introduction on Evolution of Azole Derivatives in Medicinal Chemistry” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978-981-16-8399-2_4
(78) R. Gujjarappa, A. K. Kabi, S. Sravani, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, V. Singh, S. Gupta, C. C. Malakar
“Overview on Biological Activities of Thiazole Derivatives” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978-981-16-8399-2_5
(77) R. Gujjarappa, A. K. Kabi, S. Sravani, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, R. Velayutham, V. Singh, S. Gupta, C. C. Malakar
“Overview on Biological Activities of Imidazole Derivatives” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978-981-16-8399-2_6
(76) A. K. Kabi, S. Sravani, R. Gujjarappa, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, V. Singh, S. Gupta, C. C. Malakar
“Overview on Biological Activities of Pyrazole Derivatives” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978-981-16-8399-2_7
(75) A. K. Kabi, S. Sravani, R. Gujjarappa, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, R. Velayutham, V. Singh, S. Gupta, C. C. Malakar
“An Overview on Biological Evaluation of Tetrazole Derivatives” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978-981-16-8399-2_8
(74) A. K. Kabi, S. Sravani, R. Gujjarappa, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, V. Singh, S. Gupta, C. C. Malakar
“An Overview on Biological Activity of Benzimidazole Derivatives” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978-981-16-8399-2_9
(73) R. Gujjarappa, S. Sravani, A. K. Kabi, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, V. Singh, S. Gupta, C. C. Malakar
“An overview on Biological Activities of Oxazole, Isoxazoles and 1,2,4-oxadiazoles Derivatives” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978- 981-16-8399-2_10
(73) A. K. Kabi, S. Sravani, R. Gujjarappa, A. Garg, N. Vodnala, U. Tyagi, D. Kaldhi, V. Singh, S. Gupta, C. C. Malakar
“An Overview on Biological Activities of 1,2,3-Triazole Derivatives” Springer Nature, Singapore, 2022, http://dx.doi.org/10.1007/978-981-16-8399-2_11
(72) A. K. Kabi, R. Gujjarappa, A. Garg, A. Roy, A. Sahoo, S. Gupta, C. C. Malakar
“Overview on Medicinal Impacts of 1,2,4-Triazole Derivatives” Springer Proceedings in Materials, 2022, Springer International Publishing, DOI : 10.1007/978- 981-19-2572-6_5
(71) A. K. Kabi, R. Gujjarappa, A. Garg, A. Roy, A. Sahoo, S. Gupta, C. C. Malakar
“Overview on Diverse Biological Activities of Benzisoxazole Derivatives” Springer Proceedings in Materials, 2022, Springer International Publishing, DOI: 10.1007/978-981-19-2571-9
(70) A. K. Kabi, R. Gujjarappa, A. Garg, A. Roy, A. Sahoo, S. Gupta, C. C. Malakar
“Highlights on Biological Activities of 1,3,4-Thiadiazole and Indazole Derivatives” Springer Proceedings in Materials, 2022, Springer International Publishing, DOI: 10.1007/978-981-19-2572-6
2021
(69) S. Kumar, C. C. Malakar and V. Singh
“Cu(II)-Catalysed Azide-Alkyne Cycloaddition Reaction towards Synthesis of β-Carboline C1-
Tethered 1,2,3-Triazole Derivatives”
ChemistrySelect 2021, 6, 4005-4010
(68) R. Gujjarappa, N. Vodnala, A. Kandpal, L. Roy, S. Gupta, and C. C. Malakar
“Csp-Csp Bond Cleavage and Fragments Coupling: Transition Metal-Free “Extrusion and Recombination” Approach towards Synthesis of 1,2-Diketones”
Org. Chem. Front., 2021, 8, 5389-5396
(67) A. K. Kabi, R. Gujjarappa, A. Roy, A. Sahoo, D. Musib, N. Vodnala, V. Singh, and C. C. Malakar
“Transition Metal-Free Transfer Hydrogenative Cascade Reaction of Nitroarenes with Amines/Alcohols: Redox-Economical Access to Benzimidazoles”
J. Org. Chem. 2021, DOI: https://doi.org/10.1021/acs.joc.1c01450
Publications from NIT Manipur
(66) S. Polina, V. P. R. K. Putta, R. Gujjarappa, V. Singh, P. P. Pujar and C. C. Malakar
“P(III)-Mediated Cascade C-N/C-S Bond Formation: A Protocol towards the Synthesis of N,S-Heterocycles and Spiro Compounds”
Adv. Synth. Catal. 2021, 363, 431-445
(65) S. Polina, V. P. R. K. Putta, R. Gujjarappa, P. P. Pujar and C. C. Malakar
“Aza‐Michael Addition of 1,2‐Diazoles to Structurally Diverse Enones: Efficient Methods towards β‐Amino Ketones”
J. Heterocycl. Chem. 2021, 58, DOI: 10.1002/jhet.4221
(64) V. Kumar, D. Singh, R. Gujjarappa, C. C. Malakar and V. Singh
“Efficient Approach towards the Polysubstituted 4H-Pyran Hybrid Quinolone Derivatives and Subsequent Copper-Catalyzed Hydroxylation of Haloarenes”
Heterocycles 2021, DOI: 10.3987/COM-20-14383
(63) N. Devi, A. Gupta, R. Gujjarappa, C. C. Malakar and V. Singh
“Synthesis of Substituted Pyrazolo[4,3-c]quinolones and the C-C Bond Cleavage During Reductive Cyclization”
Heterocycles 2021, DOI: 10.3987/COM-20-14403
2020
(62) V. P. R. K. Putta, N. Vodnala, R. Gujjarappa, U. Tyagi, A. Garg, S. Gupta, P. P. Pujar and C. C. Malakar
“Reagent-Controlled Divergent Synthesis of 2-Amino-1,3-Benzoxazines and 2-Amino-1,3-Benzothiazines”
J. Org. Chem. 2020, 85, 380-396. (Highlighted in Organic Chemistry Portal 2020, https://www.organic-chemistry.org/abstracts/lit7/128.shtm)
(61) R. Gujjarappa, N. Vodnala, V. G. Reddy and C. C. Malakar
“Niacin as a Potent Organocatalyst towards the Synthesis of Quinazolines using Nitriles as C-N Source”
Eur. J. Org Chem. 2020, 803-814. (Highlighted in ChemistryViews 2020, https://www.chemistryviews.org/details/ezine/11218877/Organocatalyzed_Synthesis_of_Quinazolines.html).
(60) R. Gujjarappa, N. Vodnala and C. C. Malakar
“Comprehensive Strategies for the Synthesis of Isoquinolines: Progress Since 2008”
Adv. Synth. Catal. 2020, 362, 4896-4900


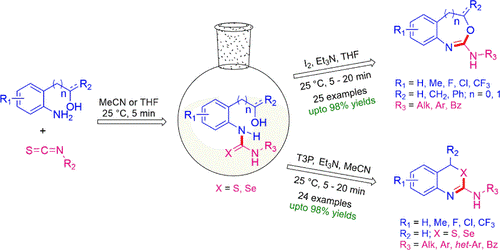














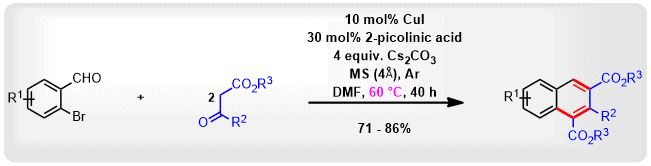

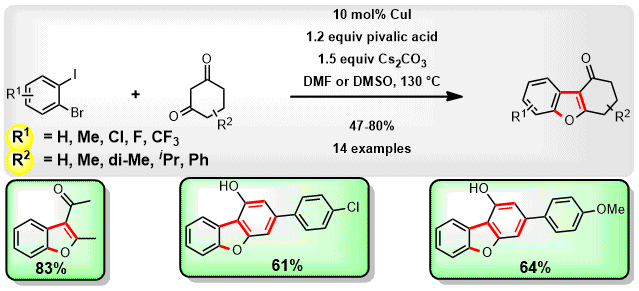
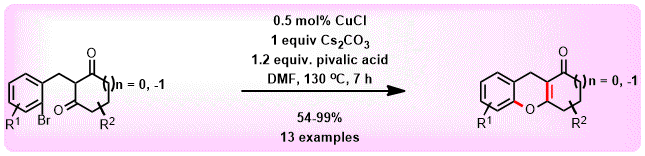
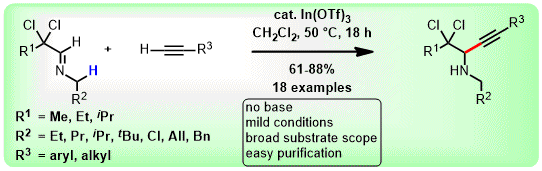








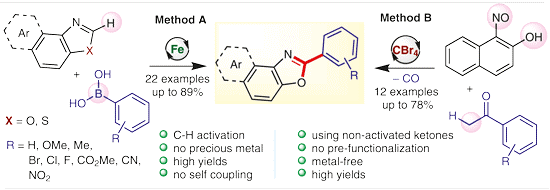










(59) S. Sharma, C. C. Malakar and V. Singh
“Transition‐Metal‐Free C‐S Bond Forming Strategy towards Synthesis of Highly Diverse Pyrazole Tethered Benzothiazoles: Investigation of their Photophysical Properties” Asian J. Org Chem. 2020, 9, 1857-1868
(58) R. Gujjarappa, N. Vodnala, V. G. Reddy and C. C. Malakar
“A Facile C-H Insertion Strategy using the Combination of HFIP and Isocyanides: The Metal-Free Access of Azole Derivatives”
Asian J. Org Chem. 2020, 9, 1793-1797
(57) N. Vodnala, R. Gujjarappa, S. Polina, V. Satheesh, D. Kaldhi, A. K. Kabi and C. C. Malakar
“Organocatalytic C-C Bond Cleavage Approach: A Metal-Free and Peroxide-Free Facile Method for the Synthesis of Amide Derivatives”
New J. Chem. 2020, 44, 20940-20944
(56) A. K. Kabi, R. Gujjarappa, N. Vodnala, D. Kaldhi, U. Tyagi and C. C. Malakar
“HFIP-Mediated Strategy towards β-Oxo Amides and Subsequent Friedel-Craft Type Cyclization to 2‑ Quinolinones using Recyclable Catalyst”
Tetrahedron Lett. 2020, 61, 152535
(55) D. Kaldhi, N. Vodnala, R. Gujjarappa, A. K. Kabi, S. Nayak and C. C. Malakar
“Transition-Metal-Free Variant of Glaser- and Cadiot-Chodkiewicz-Type Coupling: Benign Access to Diverse 1,3-Diynes and Related Molecules”
Tetrahedron Lett. 2020, 61, 151775
(54) R. Gujjarappa, N. Vodnala, V. P. R. K. Putta, V. G. Reddy and C. C. Malakar
“Conversion of Alkynes into 1,2-Diketones using HFIP as Sacrificial Hydrogen Donor and DMSO as Dihydroxylating Agent”
Tetrahedron Lett. 2020, 61, 151588
(53) R. Gujjarappa, N. Vodnala and C. C. Malakar
“Decarboxylative cyclization of amino acids towards the Regioselective synthesis of 2,4-diarylpyridines via relay Fe(III)/In(III)-catalysis”
Tetrahedron Lett. 2020, 61, 151495
(52) N. Vodnala, R. Gujjarappa, V. Satheesh, R. Gupta, D. Kaldhi, A. K. Kabi and C. C. Malakar
“Copper-Catalyzed [2+2+1+1] Annulation for the Regioselective Synthesis of 2,6-Diarylpyridines via C1-Insertion and Subsequent Cyclization”
ChemistrySelect 2020, 5, 10144-10148
(51) R. Gujjarappa, N. Vodnala, A. Garg, C. K. Hazra, S. Gupta and C. C. Malakar
“Amino Acid-Mediated Aerobic Oxidation of Organoborons for the Synthesis of Phenolic Derivatives using Single Electron Transfer”
ChemistrySelect 2020, 5, 2419-2423
(50) R. Gujjarappa, N. Vodnala and C. C. Malakar
Recent Advances in Pyridine-Based Organocatalysis and its Application towards Valuable Chemical Transformations”
ChemistrySelect 2020, 5, 8745-8758
2019
(49) R. Gujjarappa, N. Vodnala, M. Kumar, C. C. Malakar
“Pd-Catalyzed Decarboxylation and Dual C(sp3)–H Functionalization Protocols for the Synthesis of 2,4-Diarylpyridines”
J. Org. Chem. 2019, 84, 5005-5020
(48) V. P. R. K. Putta, R. Gujjarappa, U. Tyagi, P. P. Pujar and C. C. Malakar
“A metal- and base-free domino protocol for the synthesis of 1,3-benzoselenazines, 1,3-benzothiazines and related scaffolds”
Org. Biomol. Chem. 2019, 17, 2516-2528
(47) D. Singh, V. Kumar, C. C. Malakar and V. Singh
“Structural Diversity Attributed by Aza-Diels-Alder Reaction in Synthesis of Diverse Quinoline Scaffolds”
Curr. Org. Chem. 2019, 23, 920-958
(46) N. Aljaar, R. Gujjarappa, M. Al-refai, M. Shtaiwi and C. C. Malakar
“Overview on Recent Approaches towards Synthesis of 2-Keto-annulated Oxazole Derivatives”
J. Het. Chem. 2019, 56, 2730-2743
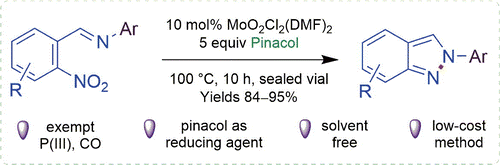
(45) D. Kaldhi, R. Gujjarappa, N. Vodnala, A. K. Kabi and C. C. Malakar
“Mo(VI)-Catalyzed Synthesis of 2-Aryl-2H-Indazoles Using Pinacol Mediated Deoxygenation of Nitroaromatics”
Chem. Lett. 2019, 48, 1258-1261
(44) D. Kaldhi, N. Vodnala, R. Gujjarappa, S. Nayak, V. Ravichandiran, S. Gupta, C. K. Hazra, C. C. Malakar
“Organocatalytic Oxidative Synthesis of C2-Functionalized Benzoxazoles, Naphthoxazoles, Benzothiazoles and Benzimidazoles”
Tetrahedron Lett. 2019, 60, 223-229
(43) N. Vodnala, R. Gujjarappa, C. K. Hazra, D. Kaldhi, A. K. Kabi, U. Beifuss and C. C. Malakar
“Copper-Catalyzed Site-Selective Oxidative C-C Bond Cleavage of Simple Ketones for the Synthesis of Anilides and Paracetamol”
Adv. Synth. Catal. 2019, 360, 135-145
2018
(42) R. Gujjarappa, S. K. Maity, C. K. Hazra, N. Vodnala, S. Dhiman, A. Kumar, U. Beifuss, C. C. Malakar
“Divergent Synthesis of Quinazolines using Organocatalytic Domino Strategies under Aerobic Conditions”
Eur. J. Org Chem. 2018, 4628-4638
(41) N. Vodnala, R. Gujjarappa, A. K. Kabi, M. Kumar, U. Beifuss and C. C. Malakar
“Facile Protocols towards C2-Arylated Benzoxazoles using Fe(III)-Catalyzed C(sp2-H) Functionalization and Metal-Free Domino Approach”
Synlett 2018, 29, 1469-1478
(40) R. Gujjarappa, N. Vodnala, A. K. Kabi, D. Kaldhi, M. Kumar, U. Beifuss and C. C. Malakar
“Efficient Syntheses of Diverse N-Heterocycles: The Molybdenum(VI)-Catalyzed Reductive Cyclization of Nitroarenes using Pinacol as a Deoxygenating Agent” SynOpen 2018, 2, 138-144
(39) D. Singh, C. K. Hazra, C. C. Malakar, S. K. Pandey, B. S. Kaith and V. Singh
“Indium-Mediated Domino Allylation-Lactonisation Approach: Diastereoselective Synthesis of β-Carboline C-3 Tethered α-Methylene γ-Butyrolactones” ChemistrySelect 2018, 3, 4859-4864
(38) D. Singh, P. Sharma, R. Kumar, S. K. Pandey, C. C. Malakar and V. Singh,
“An Expeditious Approach for the Synthesis of β-Carboline-Pyrazole-Based Molecular Hybrids”
Asian J. Org. Chem. 2018, 7, 383-394.
(37) Vipin Kumar, Sandeep Chaudhary, Manas Mathur, Ajit Kumar, Chandi C Malakar, Virender Singh, "A Tandem Approach towards Diastereoselective Synthesis of Quinoline C-3 Tethered γ-Lactones" ChemistrySelect, 2018, DOI: 10.1002/slct.201702923
(36) V.P. Rama Kishore Putta, Raghuram Gujjarappa, Nagaraju Vodnala, Richa Gupta, Prasad P. Pujar, Chandi C.Malakar, "The facile and efficient organocatalytic platform for accessing 1,2,4-selenadiazoles and thiadiazoles under aerobic conditions", Tetrahedron Letters 2018, https://doi.org/10.1016/j.tetlet.2018.01.063
2017
(35) Kalisadhan Mukherjee, Dr. Ramendra Sundar Dey, Chandi C Malakar, Biplab Paul, "A Special Section on Functional Materials for Energy and Environment: Progress and Perspective", Energy and Environment Focus, 2017, DOI: 10.1166/eef.2017.1244
(34) Dharmender Singh, Vipin Kumar, Nisha Devi, Chandi C. Malakar, Ravi Shankar, Virender Singh, "Metal–free Decarboxylative Amination: An Alternative Approach Towards Regioselective Synthesis of β‐Carboline N‐fused Imidazoles" Advanced Synthesis & Catalysis 2017, https://doi.org/10.1002/adsc.201600970
(33) N. Devi, D. Singh, G. Kaur, S. Mor, V. P. R. K. Putta, S. Polina, C. C. Malakar and V. Singh, “In(OTf)3 Assisted Synthesis of β-Carboline C-3 Tethered Imidazo[1,2-α]azine Derivatives” New J. Chem. 2017, 41, 1082-1093.
(32) Dharmender Singh, Pooja Sharma, Rakesh Kumar, Dr. Satyendra K. Pandey, Dr. Chandi C. Malakar, Dr. Virender Singh
"An Expeditious Approach for the Synthesis of β‐Carboline−Pyrazole‐Based Molecular Hybrids" Asian Journal of Organic Chemistry 2017
https://doi.org/10.1002/ajoc.201700545
2016
(31) D. Singh, V. Kumar, N. Devi, C. C. Malakar, R. Shankar, V. Singh
“Meta-free decarboxylative amination: An alternative approach towards regioselective synthesis of beta-carboline-N-fused imidazoles”
Adv. Synth. Catal. 2016, Doi: 10.1002/adsc.201600970 (In press) (Wiley-VCH, impact factor- 6.45)
(30) N. Devi, D. Singh, G. Kaur, S. Mor, V. P. R. K. Putta, S. Polina, C. C. Malakar, V. Singh
“In(OTf)3 – assisted synthesis of beta-carboline-C3-tethered imidazo[1,2-a]azine derivatives”
New Journal of Chemistry 2016, Doi: 10.1039/c6nz03210a (In press) (RSC, impact factor - 3.28)
(29) D. Singh, N. Devi, V. Kumar, C. C. Malakar, S. Mehra, R. K. Rawal, B. S. Kaith, V. Singh
“A metal free 1,3-dipolar cycloaddition approach towards regioselective synthesis of beta-carboline and isoxazole based molecular hybrid”
RSC Advances, 2016, 6, 88066 – 88076 (RSC, impact factor- 3.29)
(28) D. Singh, N. Devi, V. Kumar, C. C. Malakar, S. Mehra, S. Rattan, R. K. Rawal, V. Singh
“Natural products inspired design and synthesis of beta-carboline and gama-lactone based molecular hybrid” Org. Biomol. Chem. 2016, 14, 8154 –8166 (RSC, impact factor- 3.56)
(27) N. Devi, D. Singh, R. K. Sunkaria, C. C. Malakar, Saloni Mehra, R. K. Rawal, V. Singh,
“An In(OTf)3-HBF4 assisted multicomponent approach for one-pot synthesis of pyrrazolopyridinone fused imidazopyridines”
ChemistrySelect 2016, 1, 4696 – 4703 (Wiley-VCH)
(26) N. Vodnala, D. Kaldhi, R. Gupta, S. Polina, V.P. R. K. Putta, S. C. P. Promily, R. K. Linthoinganbi, V. Singh, C. C. Malakar “Novel Domino Routes for the Synthesis of N-Heterocycles via Reductive Cyclization of β-(N-2-Nitroaryl)-α,β-unsaturated Ketones”
ChemistrySelect 2016, 1, 5784 – 5788 (Wiley-VCH)
(25) N. Vodnala, D. Kaldhi, S. Polina, V.P. R. K. Putta, R. Gupta, S. C. P. Promily, R. K. Linthoinganbi, V. Singh, C. C. Malakar “Pd-Catalyzed Domino Reactions of Nitroaromatics: A Surrogate Access towards the Saturated N-heterocycles”
Tetrahedron Letters, 2016, 57, 5695 – 5699 (Impact factor – 2.347)
Publications from Ph.D & Postdocs
2016
(21) K. Kushwaha, B. Pinter, S. A. Shehzadi, C. M. L. Vande Velde, F. de Proft, C. C. Malakar, and K. A Tehrani, “Metal-free synthesis of chlorinated β-amino ketones via an unexpected reaction of imines with arylacetylenes in 1,1,1,3,3,3-hexafluoro-2-propanol”Adv. Synth. Catal. 2016, 358, 41-49
(20) K. Seehafer, C. C. Malakar, J. Qu and G. Helmchen, “Iridium-Catalyzed Allylic Substitutions with Bulky Amines – an Entry into the Reetz Synthesis of Aminoalcohols” Eur. J. Org. Chem. 2016, 2016, 493-501
2015
(19) N. Aljaar, C. C. Malakar, J. Conrad and U. Beifuss, “Base-Promoted Domino Reaction of 5-Substituted 2-Nitrosophenols with Bromomethyl Aryl Ketones: A Transition Metal-free Approach to 2-Ketoaryl Benzoxazoles” J. Org. Chem. 2015, 80, 10829-10837
(18) C. C. Malakar and G. Helmchen,
“Immobilized Catalyst for Iridium-Catalyzed Allylic Amination – Rate Enhancement by Immobilization”
Chem. Eur. J., 2015, 21, 7127-7134
Selected as a HOT paper and front cover page
Highlighted in Synfacts 2015, 11, 0771-0771.
Highlighted in Sigma-Aldrich Catalogue 2015, http://www.sigmaaldrich.com/catalog/papers/25787122
(17) K. Kushwaha, C. C. Malakar, S. Stas and K. A Tehrani,
“Indium(III)-Catalyzed Tandem Synthesis of 2-Alkynyl-3,3-dichloropyrrolidines via Addition/Cyclization of Alkynes to α,α,γ-Trichloroaldimines”
RSC Advances 2015, 5, 10139-10151
2014
(16) D. Schmidt, C. C. Malakar and U. Beifuss, “2,3-Dihalo-1-propenes as Building Blocks in Cu(I)-Catalyzed Domino Reactions: Efficient and Selective Synthesis of Furans” Organic Letters 2014, 16, 4862-4865.
Selected for ChemInform Abstract- ChemInform 2015, 46, Issue 12, 05 March, 2015, DOI: 10.1002/chin.201512144
2013
(15) C. C. Malakar, S. Stas, W. Herrebout and K. A Tehrani, “Lewis acid Mediated Vinyl Transfer Reaction of Alkynes to N-Alkylimines using the N-Alkyl Residue as a Sacrificial Hydrogen Donor” Chem. Eur. J., 2013, 19, 14263–14270.
Selected for ChemInform Abstract- ChemInform 2014, 45, Issue 13, 14 March, DOI: 10.1002/chin.201413075
(14) N. Aljaar, C. C. Malakar, J. Conrad, W. Frey and U. Beifuss, “The Reaction of 1-Nitroso-2-naphthols with alfa-Functionalized Ketones and Related Compounds-The Unexpected Formation of Decarbonylated 2-Substituted Naphtho[1,2-d][1,3]oxazoles” J. Org. Chem. 2013, 78, 154-166.
Selected for ChemInform Abstract- ChemInform 2013, 44, Issue 20, 25 April, DOI: 10.1002/chin.201320143
Highlighted in Sigma-Aldrich Catalog 2013 http://www.sigmaaldrich.com/catalog/papers/23181942
(13) R. K. Devappa, C. C. Malakar, H. P. S. Makkar and K. Becker, “Pharmaceutical Potential of Phorbol esters from Jatropha Curcas Oil” Nat. Prod. Res., 2013, 27, 1459-1462
(12) K. Sudheendran, C. C. Malakar, J. Conrad and U. Beifuss, “Synthesis of Functionalized Naphthalenes by Copper(I)-Catalyzed Annulation between 3-(2-Halobenzyl)pentane-2,4-diones and β-Keto Esters, Malonates and Cyanoacetates” Adv. Synth. Catal. 2013, 355, 2400-2416.
Selected for ChemInform Abstract- ChemInform 2014, 45, Issue 5, 16 Jan, DOI: 10.1002/chin.201405081
(11) A. H. Moustafa, C. C. Malakar, N. Aljaar, E. Merisor, J. Conrad and U. Beifuss, “Microwave-assisted molybdenum-catalyzed reductive cyclization of o-nitrobenzylidene amines to 2-aryl-2H-indazoles” Synlett, 2013, 24, 1573-1577.
Selected for ChemInform Abstract- ChemInform 2013, 44, Issue 46, 24 October, DOI: 10.1002/chin.201346123
2012
(10) C. C. Malakar, K. Sudheendran, H.-G. Imrich, S. Mika and U. Beifuss, “Cu(I)-catalyzed annulation for the synthesis of substituted naphthalenes using o-bromobenzaldehydes and β-ketoesters as substrates” Org. Biomol. Chem., 2012, 10, 3899-3905
Top ten most accessed article in April 2012.
Selected for ChemInform Abstract- ChemInform 2012, 43, Issue 37, 16 August, DOI: 10.1002/chin.201237081
(9) C. C. Malakar, A. Baskakova, J. Conrad and U. Beifuss, “Copper-Catalyzed Synthesis of Quinazolines in Water starting from o-Bromobenzylbromides and Benzamidines” Chem. Eur. J., 2012, 18, 8882-8885
Highlighted in Sigma-Aldrich catalog 2013, http://www.sigmaaldrich.com/catalog/papers/22730204
Selected for ChemInform Abstract- ChemInform 2012, 43, Issue 50, 29 November, DOI: 10.1002/chin.201250163
(8) N. Aljaar, C. C. Malakar, J. Conrad, S. Strobel, T. Schleid and U. Beifuss, “Cu-Catalyzed Reaction of 1,2-Dihalobenzenes with 1,3-Cyclohexanediones for the Synthesis of 3,4-Dihydrodibenzo[b,d]furan-1(2H)-ones” J. Org. Chem. 2012, 77, 7793-7803.
Selected for ChemInform Abstract- ChemInform 2013, 44, Issue 2, 09 Jan, DOI: 10.1002/chin.201302084
(7) K. Sudheendran, C. C. Malakar, J. Conrad and U. Beifuss, “Copper(I)-Catalyzed Intramolecular O-Arylation for the Synthesis of 2,3,4,9-Tetrahydro-1H-xanthen-1-ones with Low Loads of CuCl” J. Org. Chem. 2012, 77, 10194-10210.
Selected for ChemInform Abstract- ChemInform 2013, 44, Issue 2, 28 Mar, DOI: 10.1002/chin.201316120
(6) C. C. Malakar, B. U. W. Maes and K. A Tehrani, “An In(III)-Catalyzed Synthesis of 4,4-Dichloro-1-aryl-N-alkyl-1-yn-3-amines via an Intermolecular C(sp2)-C(sp) Bond Formation” Adv. Synth. Catal. 2012, 354, 3461-3467.
Selected for ChemInform Abstract- ChemInform 2013, 44, Issue 20, 25 April, DOI: 10.1002/chin.201320076
2011
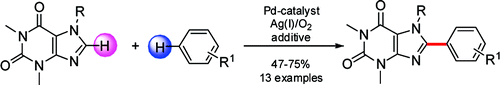
(5) C. C. Malakar, D. Schmidt, J. Conrad and U. Beifuss, “Double C-H Activation: The Palladium-Catalyzed Direct C-Arylation of Xanthines with Arenes” Organic Letters 2011, 13, 1378-1381.
Selected for ChemInform Abstract- ChemInform 2011, 42, Issue 23, 12 May, DOI: 10.1002/chin.201123153
(4) C. C. Malakar, D. Schmidt, J. Conrad and U. Beifuss, “Cu(I)-Catalyzed Domino Reactions: Efficient and Selective Synthesis of 4H-Chromenes and Naphthalenes” Organic Letters 2011, 13, 1972-1975.
Selected for ChemInform Abstract- ChemInform 2011, 42, Issue 30, 30 June, DOI: 10.1002/chin.201130140
2010
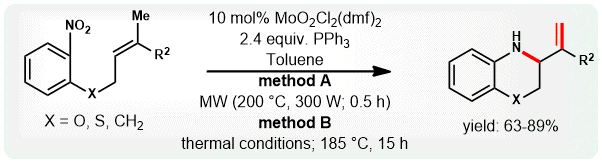
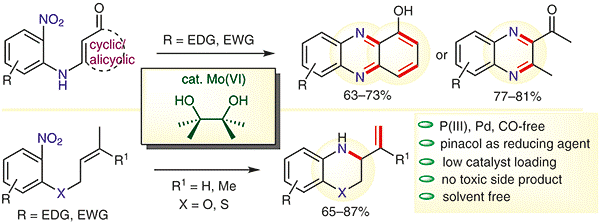
(3) C. C. Malakar, E. Merisor, J. Conrad and U. Beifuss, “MoO2Cl2(dmf)2 Catalyzed Domino Reactions of w-Nitro Alkenes to 3,4-Dihydro-2H-1,4- benzothiazines and Other Heterocycles” Synlett 2010, 1766-1770.
Highlighted in Synfacts 2010, 1123.
Selected for ChemInform Abstract- ChemInform 2010, 41, Issue 44, 7 October, DOI: 10.1002/chin.201044180
2009
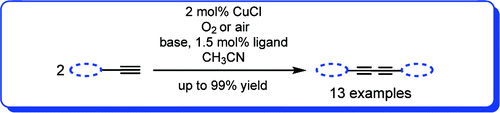
(2) S. Adimurthy, C. C. Malakar and U. Beifuss, “Influence of bases and ligands on the outcome of the Cu(I)-catalyzed oxidative homocoupling of terminal alkynes to 1,4-disubstituted 1,3-diynes using oxygen as an oxidant” J. Org. Chem. 2009, 74, 5648-5651.
Selected for ChemInform Abstract- ChemInform 2009, 40, Issue 51, 07 December, DOI: 10.1002/chin.200951078
2008
(1) E. Merisor, J. Conrad, C. C. Malakar and U. Beifuss, “Unexpected Lewis acid mediated reactions of 1-arylbut- 3-en-1-ols with trimethylorthoformate - A new synthesis of homoallyl ethers and chlorides” Synlett 2008, 903-907.
Selected for ChemInform Abstract- ChemInform 2008, 39, Issue 32, 11 July, DOI: 10.1002/chin.200832067
Conference Publications
In International conferences:
(1) E. Merisor, C. C. Malakar and U. Beifuss, “Microwave assisted cyclization of ω-nitroalkenes: Synthesis of saturated N-heterocycles” GDCH Wissenschaftsforum Chemie 16-19 Sept. 2007, Universität Ulm (Germany).
(2) C. C. Malakar, “New strategies for the transition metal-catalyzed synthesis and functionalization of carbocycles and heterocycles” 15. TAG der ORGANISCHEN CHEMIE, Stuttgart, Germany, 14 October 2011.
(3) C. C. Malakar, K. Kushwaha, W. Herrebout, K. A. Tehrani, “Lewis Acid Mediated Vinyl-Transfer Reaction of Alkynes to N-Alkylimines by Using the N-Alkyl Residue as a Sacrificial Hydrogen Donor” 17 th Sigma Aldrich meeting, 5-6 December 2013, Blankenberg, Belgium
In national conferences:
(4) C. C. Malakar, “Application of Transition metal-catalyzed Domino Reactions in the synthesis of broad range of heterocycles” 33rd Annual Conference of the Indian Council of Chemists at Department of Applied Chemistry, Indian School of Mines, Dhanbad on 15th – 17th December, 2014
(5) D. Kaldhi, N. Vodnala, R. K. Linthoinganbi, S. C. Pinky Promily, C. C. Malakar
“A Metal-free Domino Approach towards N-heterocycles using Easy Available Un-activated Simple Starting Materials”
International Conference on Nature Inspired Initiatives in Chemical Trends (NIICT), Organic Synthesi 2016, at Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad, 19 – 20 September, 2016
(6) N. Vodnala, D. Kaldhi, R. K. Linthoinganbi, S. C. Pinky Promily, C. C. Malakar
“Ferrites Makes it Possible via C(sp2)-H Activation: Towards the Development of Novel Routes for Indole Derivatives”
International Conference on Nature Inspired Initiatives in Chemical Trends (NIICT), Organic Synthesis 2016, at Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad, 19 – 20 September, 2016
(Selected for Best Poster Award)
(7) D. Kaldhi, N. Vodnala, R. K. Linthoinganbi, S. C. Pinky Promily, C. C. Malakar
“A Metal-free Approach towards C(sp)-H Functionalization of Terminal Alkynes”
International Conference on Nature Inspired Initiatives in Chemical Trends (NIICT), Organic Synthesis 2016, at Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad, 19 – 20 September, 2016
(8) N. Vodnala, D. Kaldhi, R. K. Linthoinganbi, S. C. Pinky Promily, C. C. Malakar
“A Two-fold Un-activated C(sp2)-H Activation Approach towards Synthesis of Indole Derivatives”
International Conference on Nature Inspired Initiatives in Chemical Trends (NIICT), Organic Synthesis 2016, at Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad, 19 – 20 September, 2016
(9) R. Gupta, N. Vodnala, D. Kaldhi, R. Gujjarappa, A. K. Kabi, S. K. Maity, R. K. Linthoinganbi, S. C. Pinkny Promily, V. Singh, C. C. Malakar
“A Surrogate Protocol for the Synthesis of Naphthoxazoles using Inexpensive Fe-catalyzed C- Arylation Approach” RAFAS 2016, at Lovely Professional University, Jalandhar, 25 – 26 November, 2016
(10) R. Gupta, N. Vodnala, D. Kaldhi, R. Gujjarappa, A. K. Kabi, S. K. Maity, C. C. Malakar
“Seeking towards Novel Method for the Synthesis of Heterocycles using Easy Available Nitro-compounds”
35th Annual Conference of the Indian Council of Chemists at Department of Chemistry, H. V. Desai College, Pune on 22 – 24 December, 2016





